Bioventus Reaches Milestone with 2 Million DUROLANE® Treatments Worldwide
DURHAM, NC – September 22, 2020 – Bioventus, a global leader in Innovations For Active Healing, is celebrating a milestone and recognizing more than 2 million treatments worldwide with DUROLANE, its single-injection, hyaluronic acid (HA) product used in the treatment of pain associated with osteoarthritis (OA). DUROLANE is the original single injection hyaluronic acid therapy, has helped patients in nearly 30 countries and is a trusted therapy for clinicians in the management of OA.
Proven safe and effective, DUROLANE has more clinical studies than any other US single-injection HA, and has also been proven to provide greater reduction in OA knee pain versus Synvisc-One®.1,2 It has longer lasting pain relief versus a steroid injection, is safe and repeated use of DUROLANE does not increase the incidence of adverse events.3,4
DUROLANE is available in its original 3ml syringe for treatment of large joints and in a 1ml syringe, known as DUROLANE SJ, to enable treatment of small joints.* Both are produced using a natural, safe and proven technology called NASHA®. This process yields a high molecular weight stabilized HA, which is a naturally occurring molecule that provides lubrication and cushioning in a normal joint.
“The US launch of DUROLANE in 2018 boosted our growth to more than 2 million treatments and we are very proud of this milestone. Still, there are many more patients to reach,” said John Nosenzo, Chief Commercial Officer, Bioventus. “In the US, more than 14 million people are afflicted with knee OA and osteoarthritis overall affects more than 240 million globally.5,6 Returning people to active lifestyles is our commitment to the market and we are pleased that DUROLANE is serving the needs of clinicians and the OA patients they treat.”
About Bioventus
Bioventus delivers clinically proven, cost-effective products that help people heal quickly and safely. Its mission is to make a difference by helping patients resume and enjoy active lives. The Innovations For Active Healing from Bioventus include offerings for osteoarthritis, surgical and non-surgical bone healing. Built on a commitment to high quality standards, evidence-based medicine and strong ethical behavior, Bioventus is a trusted partner for physicians worldwide. For more information, visit www.bioventus.com and follow the company on LinkedIn and Twitter.
Media Contact: Thomas Hill, , [email protected]
Bioventus, the Bioventus logo, and DUROLANE are registered trademarks of Bioventus LLC. NASHA is a registered trademark. Synvisc-One and Synvisc are registered trademarks of Genzyme Corporation.
*DUROLANE SJ is not available in the US.
Summary of Indication for Use: DUROLANE is indicated for the treatment of pain in osteoarthritis (OA) of the knee in patients who have failed to respond adequately to conservative non-pharmacological therapy or simple analgesics, e.g. acetaminophen. Do not inject DUROLANE in patients with knee joint infections, skin diseases, or other infections in the area of the injection site. Do not administer to patients with known hypersensitivity or allergy to sodium hyaluronate preparations. Risks can include transient pain or swelling at the injection site. DUROLANE has not been tested in pregnant or lactating women, or children. Full prescribing information can be found in product labeling, at www.DUROLANE.com, or by contacting Bioventus Customer Service at 1-800-836-4080.
NOTE: The above indications presented are for the US market; indications may vary by country. Contact your local Bioventus representative or visit www.durolane.com/uk/selection for prescribing information specific to your market.
- McGrath AF, McGrath AM, Jessop ZM, et al. A comparison of intra-articular hyaluronic acid competitors in the treatment of mild to moderate knee osteoarthritis. J Arthritis. 2013; 2(1):108. doi:10.4172/2167-7921.1000108. 2. DOF RPT-001111 3. Leighton R, Åkermark C, Therrien R, et. al. NASHA hyaluronic acid vs methylprednisolone for knee osteoarthritis: a prospective, multi-centre, randomized, non-inferiority trial. Osteoarthritis Cartilage. 2014; 22(1):17-25. 4. DUROLANE [package insert]. Durham, NC: Bioventus LLC; 2017. 5. Arthritis Foundation. What is osteoarthritis? Accessed February 9, 2019. www.arthritis.org/about-arthritis/types/osteoarthritis/what-is-osteoarthritis.php 6. Hawker GA. Osteoarthritis is a serious disease. Clin Exp Rheumatol. 2019;37 Suppl 120(5):3-6.
Bioventus Reports First Use of its SIGNAFUSE Bioactive Bone Graft in Strip Format
DURHAM, NC – September 2, 2020 – Bioventus is sharing results of the first in-patient use of its new SIGNAFUSE Bioactive Bone Graft strips. Rick Placide, M.D. of VCU Health in Richmond, VA, performed a posterolateral lumbar fusion (PLF) and placed the SIGNAFUSE strips in the posterolateral gutters of the patient’s spine.
“Since the recent release of the SIGNAFUSE strip, we have used it as a bone graft extender in several cases and found it easy to use,” remarked Dr. Placide. “We have already been impressed by its ability to absorb bone marrow aspirate while maintaining its form, flexibility and toughness. These properties make SIGNAFUSE strips ideal for a variety of cases, including spanning large defects. We look forward to following the clinical outcomes.”
“SIGNAFUSE strips were designed with the patient in mind. They come in multiple sizes ranging from 25 to 200 millimeters in length, allowing utilization over multiple levels like we saw in Dr. Placide’s deformity application case,” said Lawrence Boyd PhD, Vice President, Product Development, Bioventus. “There are benefits for the surgeon as well: these strips hydrate rapidly and provide exceptional handling properties for intraoperative flexibility.”
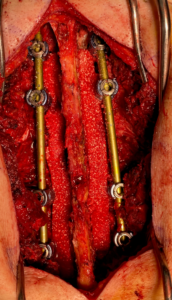
The new strip format of SIGNAFUSE is available now from Bioventus distributors nationwide.
About Bioventus
Bioventus delivers clinically proven, cost-effective products that help people heal quickly and safely. Its mission is to make a difference by helping patients resume and enjoy active lives. The orthobiologic products from Bioventus include offerings for osteoarthritis, surgical and non-surgical bone healing. Built on a commitment to high quality standards, evidence-based medicine and strong ethical behavior, Bioventus is a trusted partner for physicians worldwide. For more information, visit www.bioventus.com and follow the company on LinkedIn and Twitter.
Media Contact: Thomas Hill, , [email protected]
Bioventus, the Bioventus logo and SIGNAFUSE are registered trademarks of Bioventus LLC.
Summary of Indications for Use
SIGNAFUSE Bioactive Bone Graft is a bone graft substitute intended for use in bony voids or gaps of the skeletal system not intrinsic to the stability of the bony structure. These osseous defects may be surgically created or result from traumatic injury to the bone. SIGNAFUSE Bioactive Bone Graft is indicated to be combined with autologous bone marrow aspirate and packed into osseous defects of the extremities, pelvis and posterolateral spine. When used in the posterolateral spine, SIGNAFUSE Bioactive Bone Graft is to be used as an autograft extender. The device resorbs and is replaced by host bone during the healing process.

